What is COBRA? Is it a snake? A crisis meeting held in the Cabinet Office Room A? A beer?
No – it’s way more important.
Clinical Outcome Assessment in Brain Tumour Trials (COBRA)
COBRA is a study that is well underway and due to be completed later this year. Brain cancer studies traditionally focus on tumour responses and overall survival. But there is growing recognition that studies need to consider high quality information about outcomes such as quality of life (QoL) and function, important to those living with a brain tumour. This is particularly relevant where survival benefits are modest. Results can then be related to patients’ own lived experience, allowing informed choices about care.
What are Patient Reported Outcomes?
Patient Reported Outcomes (PROs) are measures which are directly reported by patients and which reflect their personal experience of their disease and its treatment. They constitute one component of a group of patient focused measures collectively termed Clinical Outcome Assessments (COAs). Clinician Reported Outcome (ClinRO) measures, Observer Reported Outcome (ObsRO) measures and Performance Outcome (PerfO) measures are others (see figure 1). Use of PROs allows assessment of trade-offs between tumour responses and effects on patients.
We know that:
- many brain tumour trials gather PRO information but the vast majority either never report it, or don’t assess its importance alongside tumour response
- there’s no consensus on types of patient outcomes assessed, negating opportunities to compare results between studies, or combine powerful information across studies.
To remedy this, consensus is being sought from patients, families and researchers about essential domains of patient/family experience to be collected across every glioma (grades 2-4) trial. The consensus is producing a patient-oriented Core Outcome Set (COS) applied across UK studies. COS development in other conditions has improved the quality and consistency of information captured, facilitating data sharing to produce practice-changing results.
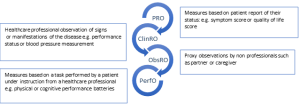
Figure 1. Examples of Clinical Outcome Assessments. COAs are based on patient, professional or carer assessment and interpretation.
Why do we need this research?
The Brain Tumour JLA Priority Setting Partnership gives context to the priority of clinical research in this area. As such, there is increasing public and healthcare recognition of the need for interventions to demonstrate improvements in patient focused outcomes, such as symptoms, physical or cognitive function and quality of life.
In addition, opportunities for public involvement in brain cancer studies are increasing with initiatives including the Tessa Jowell Brain Cancer Mission and the Tessa Jowell BRAIN-MATRIX study. It’s therefore more important than ever that individual studies capture the same core outcomes reported by, and most relevant to, patients/families. This will lead to the development of a comprehensive framework for patient centred outcomes within brain tumour studies.
References
- Top 10 priorities for clinical research in primary brain and spinal cord tumours. Final Report of The James Lind Alliance Priority Setting Partnership in Neuro Oncology. June 2015.
- Armstrong TS, Gilbert MR. Net clinical benefit: functional endpoints in brain tumor clinical trials. Curr Oncol Rep 2007;9: 60–65.
- Patient-Centered Outcomes Research Institute. Patient-centered outcomes research definition. Response to public input. Consensus definition as of Feb 15, 2012. Washington DC: Patient-Centered Outcomes Research Institute, 2012. http://www.pcori.org/assets/PCOR-Definition-Revised-Draft-and-Responses-to-Input.pdf (accessed 2 Jan 2019)
If you would like to use your experience and insight to support clinical research, consider signing up to be a PRIME advocate for brainstrust.
If you or someone you love is living with a brain tumour and have any questions around this latest news, or want to access support, give us a call on 01983 292 405 or email hello@brainstrust.org.uk. You can also visit our little brainstrust website which features support for children affected by brain tumour.